Pipeline
Adeira Pharma has developed two early preclinical candidates that we are seeking to partner for late-stage development and clinical trials.
Paclitaxel, a widely used cancer drug, is a notoriously difficult to formulate small molecule therapeutic. Much of its toxicity in patients derives from Cremophor-EL, a solvent used to dissolve paclitaxel in aqueous media such as blood plasma. Not a true excipient, Cremophor EL exerts many unwanted biological effects that render its use in formulating the taxanes (and other poorly water-soluble small molecules) less than ideal. Modified formulations, such as Abraxane, have improved the toxicity profile, but additional improvements in biodistribution and efficacy are needed.
Using a proprietary combination of lipids, and mAb targeting, we have developed a new formulation of paclitaxel that has shown very strong efficacy with low toxicity in mouse xenograft models of several different cancers.
In addition, early PK studies suggest a much longer circulation half-life and area under the curve (AUC). Extensive bioanalytical studies consistently demonstrate that the overwhelming majority of the paclitaxel is contained in the nanoparticles, with negligible or undetectable amounts residing in the aqueous phase. The following plot shows the results of one study in an MDA-MB231 breast cancer model. Other studies in this and other cancers show equally robust responses.
Further information is available upon request, and samples for analysis will be provided under a mutually suitable materials transfer agreement.
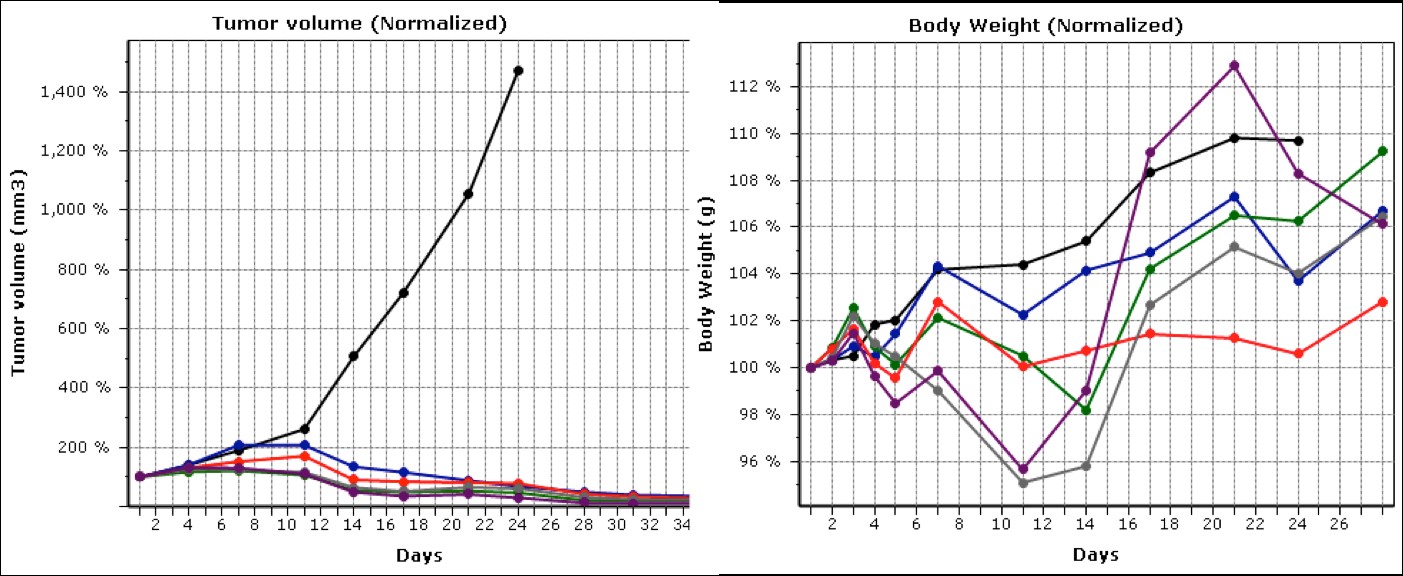
Tumor volume and body weights of an MDA-MB231 breast cancer mouse xenograft model. The mice were dosed every othe day for a total of five doses, over a range of 20-40 milligrams per kilogram of body weight. The black lines in both graphs represent the negative control group.
Adeira Pharma has several other formulations for licensing that have encouraging preliminary data. These formulations include cisplatin, carboplatin, docetaxel, and a co-formulation that incorporates both cisplatin and paclitaxel in the lipid nanoparticles.
These formulations have been evaluated for particle size and dispersion, stability, quantity of the active pharmaceutical ingredient(s), and early MTD and efficacy data in animal models of cancer. Further information is available upon request, and samples for analysis will be provided under a suitable materials transfer agreement.
For questions and inquiries regarding licensing, please email us at info@adeirapharma.com.
